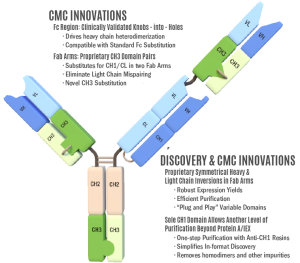
Invenra’s T-Body™ Trispecific Antibody Platform targets three distinct antigens simultaneously, offering a powerful solution for complex diseases by integrating three Fab arms into a single IgG-like structure. This innovative format enhances therapeutic potential through receptor clustering, target-specific engagement, and novel immunomodulatory mechanisms.
Why Trispecific Antibodies?
Trispecific antibodies (tsAbs) represent a next-generation modality that addresses the limitations of monospecific and bispecific antibodies by allowing simultaneous engagement with three unique targets. This approach can:
- Increase target specificity
- Reduce antigen escape
- Amplify receptor clustering and internalization
- Enhance immunostimulatory effects
Engineered for Robust
Expression & Purity
Our T-Body platform incorporates proprietary CH3 domain pairs engineered for modular, plug-and-play assembly. By substituting CH1/CL with these specialized CH3 domains, we achieve:
- High expression yields in HEK and CHO cells, consistently exceeding 100 µg/mL
- Single-step purification using CH1 resin, delivering >90% purity
- Flexible Fab domain pairing with kappa and lambda light chains
- Significantly reduced misassembly products
Optimized Manufacturing and CMC
The T-Body platform is designed to align with standard antibody manufacturing workflows:
- Protein A and Ion Exchange polishing
- Anti-CH1 resin for one-step purification
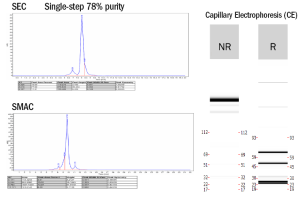
- Low aggregate formation confirmed by SEC and CE-SDS
T-Body Binding Kinetics
Binding kinetics is crucial in evaluating the therapeutic potential of trispecific antibodies, as it determines the strength, duration, and specificity of antigen-antibody interactions. Our T-Body platform has been thoroughly investigated with biolayer interferometry (BLI) and surface plasmon resonance (SPR) to assess the simultaneous binding of three distinct antigens. Key kinetic parameters include:
- Association Rate (ka): Measures how quickly the antibody binds to the target antigen.
- Dissociation Rate (kd): Indicates how rapidly the antibody-antigen complex dissociates.
- Equilibrium Dissociation Constant (KD): Reflects the binding affinity, calculated as kd/ka. Lower KD values indicate stronger binding affinity.
In the T-Body platform, each Fab arm’s binding kinetics are independently assessed to ensure optimal affinity and specificity for each target antigen. This approach provides a comprehensive view of multi-target engagement, enhancing therapeutic efficacy.
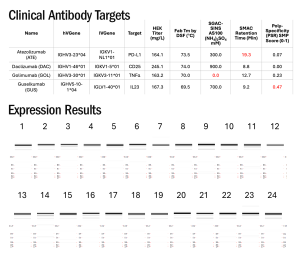
T-Body Performance: Superior Yield with Challenging Antibodies
Four clinical antibodies with diverse germlines and developability challenges (Jain et al., PNAS, 2017) were selected for a matrixing experiment, with 3 antibodies having kappa LCs and 1 antibody having a lambda LC. All possible arrangements of these domains results in 24 T-Bodies.
Expression results from the T-Body matrix experiment demonstrate the excellent performance of platform. Expi-CHO expression followed by one-step CH1 purification resulted in yields ranging from 70-340 mg/L, with 75–95% purity as assessed by non-reducing CE-SDS.